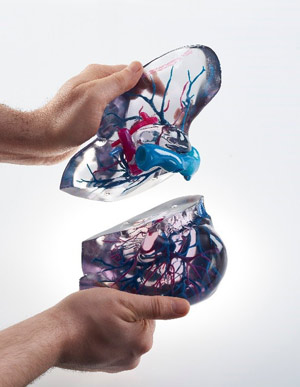
We now consider the hospital opportunity to take advantage of 3D printing to improve patient care, research, education and training, and overall hospital efficiency.
3D Printing in Hospitals
Stratasys customers in hospitals are implementing 3D printing in a wide range of uses cases: to produce patient-specific medical models for procedural planning; anatomy education of medical students; procedural simulation and training for students and physicians in continuing medical education; fabrication of highly complex research jigs and fixtures; and development of prototypes of new medical devices invented in the hospital. For the vast majority of these use cases, there are no regulatory concerns – the notable exceptions are for emergency use clearance of prototype inventions, and for prototypes of medical innovations intended for use in patients under an IRB process in the hospital.
Per the FDA, 3D printed surgical procedural models are not regulated medical devices – Scott Rader Tweet thisThe one remaining use case with regulatory considerations for 3D printing in hospitals today is taking digital patient information from CT, MRI or ultrasound scans and converting it into a 3D print to allow physicians to prepare for complex procedures. As noted previously in part 1, the rate of peer-reviewed publications for this application is growing exponentially. In addition to the data on the rate of publication, our commissioned analysis highlighted important impact factors on why physicians are turning to 3D printed medical models to:
- COMMUNICATE: Educate patients and caregivers on the surgical target and procedure plan in order to gain informed consent, as well as to better coordinate complex procedures with a multi-disciplinary surgical team.
- PLAN: Assess patient anatomy to determine the primary procedure plan and investigate back-up plans that might be required.
- PRACTICE: Dissect or manipulate models to rehearse the procedure before entering the operating room.
- DETERMINE: Ascertain the appropriate intervention, including rule-in or rule-out decisions on intervention, products or therapies chosen before committing the patient to the proposed options.
These are extremely important value propositions for patients and care-givers, and in a future blog post we will cover the more detailed published results on the impact on clinical outcomes and the economics of care.
On the regulatory front, the FDA authors’ article clearly stated that procedural planning medical models are not regulated medical devices, and the 3D printers are not a focus of regulation, but the software to prepare digital data from patient scans is a regulated product. As the paper’s authors state, “Software has been cleared through the 510(k) pathway that allows for segmentation of 3D patient scans, for example CT or MRI scans, to be converted to a 3D representation of the anatomy. This type of file, such as an STL, is similar to outputting an image as a PDF. The software used to generate the 3D model of the patient anatomy is evaluated by the FDA to assess the accuracy of the 3D volume reconstructed from image slices; however, the printer used to print the 3D component is outside of the scope of FDA review, much like an office’s laser printer would be when printing a PDF image. Prints of patient anatomy should be unaltered by the software if they are intended to be used for diagnostic or clinical purposes.”
In hospitals, the regulatory considerations for the use of 3D printing and additive manufacturing to make medical models is clear. In addition, we have witnessed emergency clearance from the FDA for clinical use of 3D printed bio-absorbable trachea scaffolds for the treatment of tracheomalacia in babies. What is not covered in the May article, but a question we have heard frequently from our customers, is: “What are the challenges for the future?”
The Future – a Start to Thinking Ahead
From the data so far, the regulatory processes known today are clear for medical products made with additive manufacturing at compliant factory facilities and medical models 3D printed at hospitals.
What has not been tackled so far is a fairly obvious question: What if we want to take the promise of the digital framework of Industry 4.0 forward to the point of combining patient-specific data with remote design, immediate digital data transfer to a 3D printer at a remote site like a hospital, and using additive manufacturing at the hospital? What are the ramifications, and the concomitant planning, that we should start thinking about now, in order to realizing the potential of making products in a more distributed fashion?
The value proposition of this framework – the ability to fulfill products at the site of care, at the time of visit, without shipping delays or inventory carrying costs – has powerful transformational potential for the business of healthcare provision.
The challenges I see on the road to this vision include the following:
- The FDA has the right to inspect manufacturing facilities with announced and un-announced visits, with significant information request rights.
- If a remote facility, up to and including a hospital, is the site of manufacture, who will have the required information ready for the FDA?
- Who will be the “responsible person” that the FDA engages at each remote site with a 3D printer or printers?
- How will quality systems need to morph to support distributed manufacturing?
- How are remote processes kept in control?
- Can automated systems be designed to allow digital capture of manufacturing verification data, data collation and evidence capture proving maintenance of product quality?
- Who will be the Responsible Person at remote locations?
- How do we engage the FDA to discuss the advantages to patients and providers of this product fulfillment vision, and together define the hurdles that need to be surmounted? As the authors note in their May article, the FDA has been engaged with manufacturers and other stakeholders and remains a willing partner.
My personal opinion is that if we can define the challenges, the entire ecosystem of innovative companies across product lifecycle management systems, quality control systems, inspection and test products, imaging and PACS systems, and additive manufacturing can come together to create robust solutions.
Follow Stratasys Healthcare on our LinkedIn Showcase page
Stratasys remains committed to being a medical additive manufacturing solutions provider, and as a leader in the industry we are pursuing and executing in partnerships like these to unlock the future transformative power of additive manufacturing.
Conclusions
The data is clear – there is an exponential increase in the adoption of 3D printing and additive manufacturing, from medical product makers to hospitals. The FDA has been a proactive partner to ensure patient safety, is working with medical innovators, and has cleared or approved a broad range of devices that fit within known ecosystems of cGMP compliance.
For me, these hopeful signs have allayed the concerns raised in worried emails I receive that state that there are “unknown” problems on the road to using additive manufacturing in medicine. The guidelines are clear, and, for the future, the 3D printing industry will need to take the lead to be proactive in designing new industrial frameworks that span more than a single niche application in order to provide comprehensive solutions covering the entire manufacturing ecosystem.
Click here to stay on top of 3D printing innovation in healthcare